Introduction :
High-Grade-B-cell Lymphoma with MYC and BCL2 and/or BCL6 rearrangements (HGBL double hit [HGBL-DH] or HGBL triple hit [HGBL-TH]), or not otherwise specified (HGBL NOS), are distinct subtypes among Large B-Cell lymphoma (LBCL) considered to be more aggressive diseases. These entities are often resistant to standard chemotherapy (Landsburg, JCO, 2017). CAR-T cell therapy (CAR-T) have changed the treatment landscape of LBCL in chemo resistant LBCL in 3 rd line, and more recently in 2 nd line of treatment. Prognosis with CAR-T seems to be as good in HGBL as in other LBCL subtypes. (Neelapu, NEJM, 2017; Schuster, NEJM, 2019; Zurko, ASH 2022). However, Fluorescence In Situ Hybridation (FISH) study isn't systematically done at diagnosis leading to an underestimation of the diagnosis of HGBL. The aim of this study was to compare the prognosis of a large cohort of patients with HGBL versus other LBCL subtypes, characterized with FISH at diagnosis, in a real life setting, based on the French DESCAR-T registry (NCT04328298).
Methods :
We collected clinical and pathological data of patients treated for relapsed/refractory LBCL or HGBL in third line or more. Data were collected both at the time of the decision of treatment in multidisciplinary meeting (MDM) and at the time of CAR-T cell infusion. Histology other than de-novo LBCL were excluded. All patients should have been studied with FISH at least with MYC probe. If a MYC rearrangement was identified, a BCL2 and/or BCL6 rearrangement should have been characterized. A p-value <0.05 was significant.
Results :
Between January 2018 and November 2022, 228 patients meeting the inclusion criteria were included across 14 centers, 73 with HGBL (28 HGBL-DH with MYC- BCL2 rearrangement, 8 HGBL-DH with BCL6 rearrangement, 14 HGBL-TH, 23 HGBL NOS) and 155 with non-HGBL (LBCL with [n=19] or without [n=136] MYC rearrangement). Characteristics of patients with HGBL vs non-HGBL at eligibility for CAR-T and at infusion of CAR-T cells were similar with no statistical difference.
At the time of CAR-T treatment decision, median age was 62 years and the others characteristics were as follows in patients with HGBL vs patients with non-HGBL : ECOG>=2: 9 (12 %) vs 23 (15%), LDH > 1N : 52 (71%) vs 119 (77%) and Ann Arbor stage III-IV : 63 (86%) vs 125 (81%). Median number of prior lines of treatment was 2 (maximum 7 in HGBL vs maximum 9 in non-HGBL).
Among the population, 60 (82%) HGBL and 135 (87%) non-HGBL patients received CAR-T cell therapy. In non-treated patients : 5 (39%) vs 8 (40%) had a disease progression and 3 (23%) vs 7 (35%) died. In treated patients, 55 (92%) HGBL vs 126 (93%) non-HGBL received at least one bridging therapy before CAR-T infusion ; and response to bridging therapy was similar among them : 19 (35%) patients with HGBL were in complete or partial response vs 46 (37%) with non-HGBL.
Characteristics of treated patients at time of infusion were also similar and as follows in HGBL patients vs non-HGBL patients : 16 (27%) vs 34 (25%) had bulky disease and 16 (27%) vs 31 (23%) had an elevated CRP. 23 (38%) vs 45 (33%) were treated with Tisa-cel and 90 (67%) vs 37 (62%) were treated with Axi-cel.
The frequency of Cytokine Release Syndrome (CRS) and Immune effector-Cell Cytotoxicity Syndrome (ICANS) (including grade >=3) did not significantly differ : 56 (93%) patients with HGBL vs 114 (84%) patients with non-HGBL presented CRS and 23 (38%) vs 55 (41%) presented ICANS. Interestingly, more patients were admitted in ICU in the HGBL cohort : 21 (35%) vs 23 (17%) (p=0.009). Macrophage Activation Syndromes was reported for 2 HGBL patients (3%).
Median follow-up was 20.5 [95 CI, 19.2-25.5] months from eligibility decided in MDM and 18.5 m [95CI, 14.3-23.4] from infusion.
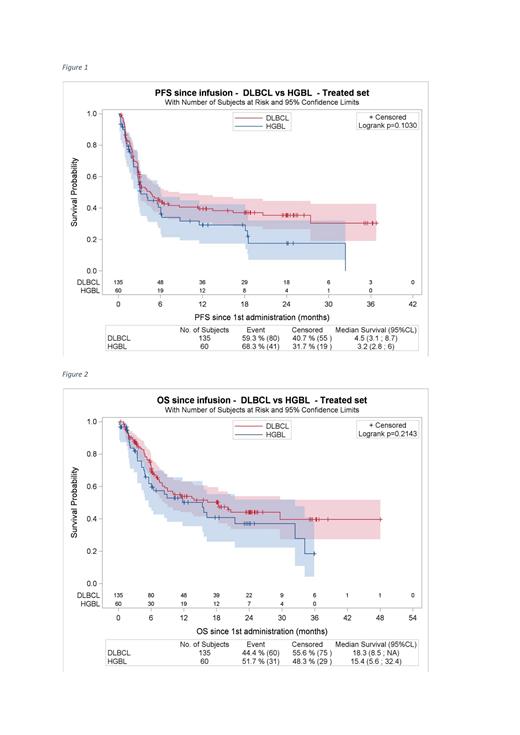
Progression free survival (PFS) and Overall survival (OS) were similar in both groups. Since eligibility, median OS was 7.6 m [95 CI, 6.4-18.3] for HGBL patients vs 11.8 m [95 CI, 9.5-22] for non-HGBL (p = 0.062). Since infusion, median PFS was 3.2 m [95CI, 2.8-6.0] for HGBL patients vs 4.5 m [95CI, 3.1-8.7] for non-HGBL (p = 0.103) Figure 1 and median OS was 15.4 m [95CI, 5.6-32.4] for HGBL patients vs 18.3 m [95CI, 8.5-not reached] for non-HGBL (p = 0.214). Figure 2
Conclusion :
CAR-T cell therapy used in third line or more seems to overcome the poor prognosis of HGBL subtypes as compared to other LBCL subtypes, all characterized with FISH. This observation supports the interest to evaluate the potential benefit of CAR-T earlier in the course of the disease.
Disclosures
Bachy:Pfizer: Honoraria, Other: Personal Fees; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Novartis: Honoraria, Other: Personal Fees; Incyte: Honoraria; Takeda: Honoraria; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Amgen: Research Funding; Kite, a Gilead Company: Honoraria, Other: Personal Fees; Roche: Consultancy, Honoraria. Gros:Kitegilead: Consultancy, Other: Consulting fees, travel and accomodation expanses; B.M.S: Consultancy, Other: Consulting fees; Milteny: Consultancy, Other: Consulting fees; Novartis: Consultancy, Other: Consulting fees, travel and accommodation expanses. Di Blasi:Kite Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; BMS: Consultancy; Pfizer: Honoraria; Incyte: Honoraria; abbvie: Honoraria; Janssen: Consultancy. Herbaux:AbbVie, Takeda: Research Funding; Physician and professor of Hematology at academic center (CHU Montpellier France): Current Employment; AbbVie, F. Hoffmann-La Roche Ltd, AstraZeneca, Janssen: Consultancy; AbbVie, F. Hoffmann-La Roche Ltd, AstraZeneca, Janssen: Honoraria. Carras:Janssen Cilag: Membership on an entity's Board of Directors or advisory committees, Other: travel fees, Research Funding; Astrazeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Other: Travelfees; Beigene: Membership on an entity's Board of Directors or advisory committees; Kitegilead: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bories:Kite Gilead: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Casasnovas:Roche: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; ADC therapeutics: Consultancy, Honoraria. Jardin:Janssen, Gilead, AbbVie, F. Hoffmann-La Roche Ltd, BMS, Takeda: Honoraria. Morschhauser:F. Hoffmann-La Roche Ltd, Gilead, AbbVie: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd, AbbVie, BMS, Genmab, Gilead, Novartis: Consultancy. Roulin:Kitegilead: Other: Conference speaker and travel grants, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Scientific advisory board; BMS: Consultancy, Other: Conference speaker, Speakers Bureau.